Paper on new microfluidics device that can detect cancer cells wins award

Main news story
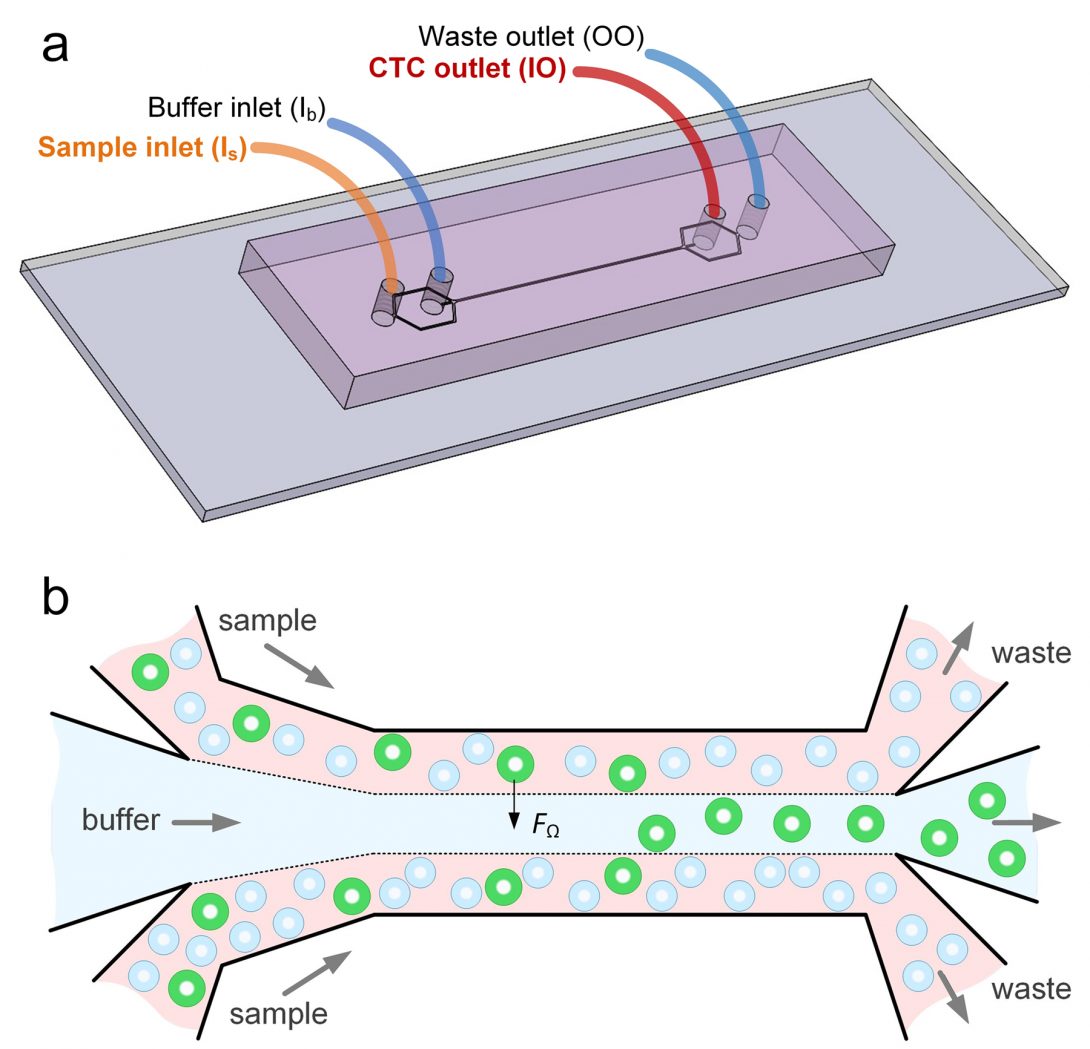
Researchers at the University of Illinois Chicago and Queensland University of Technology of Australia, have developed a device that can isolate individual cancer cells from patient blood samples. The microfluidic device works by separating the various cell types found in blood by their size. The device may one day enable rapid, cheap liquid biopsies to help detect cancer and develop targeted treatment plans. The findings are reported in the journal Microsystems & Nanoengineering.
The publication announced in August that the paper received its outstanding paper award for this year. Microsystems & Nanoengineering said the authors will be presented with an award certificate and an award prize by Springer Nature and Chinese Academy of Sciences.
“This new microfluidics chip lets us separate cancer cells from whole blood or minimally-diluted blood,” said Ian Papautsky, the Richard and Loan Hill Professor of Bioengineering in the UIC College of Engineering and corresponding author on the paper. “While devices for detecting cancer cells circulating in the blood are becoming available, most are relatively expensive and are out of reach of many research labs or hospitals. Our device is cheap, and doesn’t require much specimen preparation or dilution, making it fast and easy to use.”
The ability to successfully isolate cancer cells is a crucial step in enabling liquid biopsy where cancer could be detected through a simple blood draw. This would eliminate the discomfort and cost of tissue biopsies which use needles or surgical procedures as part of cancer diagnosis. Liquid biopsy could also be useful in tracking the efficacy of chemotherapy over the course of time, and for detecting cancer in organs difficult to access through traditional biopsy techniques, including the brain and lungs.
However, isolating circulating tumor cells from the blood is no easy task, since they are present in extremely small quantities. For many cancers, circulating cells are present at levels close to one per 1 billion blood cells. “A 7.5-milliliter tube of blood, which is a typical volume for a blood draw, might have ten cancer cells and 35-40 billion blood cells,” said Papautsky. “So we are really looking for a needle in a haystack.”
Microfluidic technologies present an alternative to traditional methods of cell detection in fluids. These devices either use markers to capture targeted cells as they float by, or they take advantage of the physical properties of targeted cells — mainly size — to separate them from other cells present in fluids.
Papautsky and his colleagues developed a device that uses size to separate tumor cells from blood. “Using size differences to separate cell types within a fluid is much easier than affinity separation which uses ‘sticky’ tags that capture the right cell type as it goes by,” said Papautsky. “Affinity separation also requires a lot of advanced purification work which size separation techniques don’t need.”
The device Papautsky and his colleagues developed capitalizes on the phenomena of inertial migration and shear-induced diffusion to separate cancer cells from blood as it passes through ‘microchannels’ formed in plastic. “We are still investigating the physics behind these phenomena and their interplay in the device, but it separates cells based on tiny differences in size which dictate the cell’s attraction to various locations within a column of liquid as it moves.”
Papautsky and his colleagues ‘spiked’ 5-milliliter samples of healthy blood with 10 small-cell-lung cancer cells and then ran the blood through their device. They were able to recover 93 percent of the cancer cells using the microfluidic device. Previously-developed microfluidics devices designed to separate circulating tumor cells from blood had recovery rates between 50 percent and 80 percent.
When they ran eight samples of blood taken from patients diagnosed with non-small-cell lung cancer, they were able to separate cancer cells from six of the samples using the microfluidic device.
In addition to the high efficiency and reliability of the devices, Papautsky said the fact that little dilution is needed is another plus. “Without having to dilute, the time to run samples is shorter and so is preparation time.” They used whole blood in their experiments as well as blood diluted just three times, which is low compared to other protocols for cell separation using devices based on inertial migration.
Papautsky and colleague Dr. Alicia Hubert, assistant professor of surgery in the UIC College of Medicine, recently received a $125,000, one-year grant from the University of Illinois Cancer Center to develop a microfluidics device that can separate out circulating tumor cells as well as detect DNA from cancer cells in blood from lung cancer patients. They will use blood from patients being seen at the University of Illinois Cancer Center to test the efficacy of their prototype device.
Jian Zhou and Amanda Bogseth from the University of Illinois Chicago and Arutha Kulasinghe, Ken O’Byrne and Chamindie Punyadeera from Queensland University of Technology, Australia, are co-authors on the paper.
This research was supported in part by the Richard and Loan Hill Department of Bioengineering at the University of Illinois Chicago and by Cancer Australia Grant APP1145657.