Large NIH grant seeks to chip away at Alzheimer’s mystery
Text block one
Though vast sums have been raised to help scientists understand and cure Alzheimer’s disease, which affects more than 5 million Americans, many of the key characteristics of this brain illness remain a mystery.
Two professors in the Richard and Loan Hill Department of Bioengineering at UIC are hoping to unlock some of these mysteries with a new grant of nearly $2 million from the National Institute on Aging of the National Institute of Health.
Researchers in Associate Professor James Lee’s Cell Mechanics and Signaling Laboratory for Neurodegenerative Diseases and Richard and Loan Hill Professor of Bioengineering Xincheng Yao’s Biomedical Optics and Functional Imaging Laboratory plan to explore the relationship between tau proteins and amyloid-β peptides, also known as Aβ, and the Alzheimer’s disease (AD) brains.
Lee said recent research has found that tau proteins physiologically exist in fluid outside the cells, such as in the blood circulating in the body of Alzheimer’s patients, and that the spread of tau proteins in the brain has related to the progression of the disease. But researchers do not understand how tau proteins interact with the blood-brain barrier, which is supposed to protect and isolate the brain from the circulating blood in the body.
“Whether and how the interactions of tau with brain cells affect cell function have yet to be fully explained,” Lee said. “We will study the underlying mechanisms and mechanical consequences resulted from the interactions of tau with brain microvascular cells of the blood-brain barrier model, and the alterations of the retinal microvascular network in [Alzheimer’s disease] mouse models expressing human Aβ and tau.”
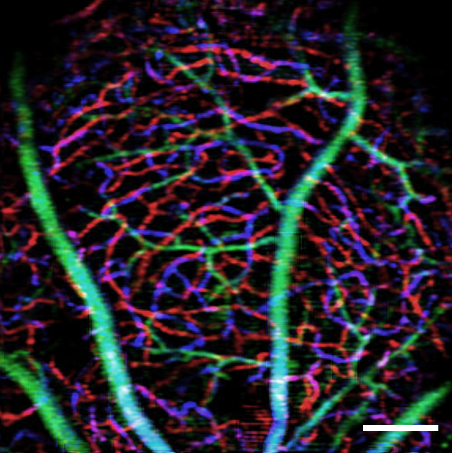
To conduct the study, Lee’s lab will use biochemical techniques and atomic force microscopy to study cell signaling and cell mechanic alterations induced by tau proteins in brain microvascular cells in a blood-brain-barrier model. Yao’s lab will use optical coherence tomography (OCT) and OCT angiography to study alterations in retinal microvascular network and neurodegeneration in Alzheimer’s disease mouse models. The team is targeting the retina because it can be used as a window of the brain for studying the disease non-invasively while the experimental subjects are still alive.
Lee stressed that Alzheimer’s is an incredibly complex illness and it is unlikely that any one intervention well be found to delay, prevent, or cure it. But each new piece of research helps to fill in the puzzle of how the disease works and affects people.
“This project will help fill a research gap between tau proteins found outside of cells and neurovascular abnormalities seen in Alzheimer’s patients by illuminating the biochemical and adhesion mechanical responses to tau in brain microvascular cells and microvascular network characteristics and neurodegeneration in relations to Aβ and tau in the retina,” Lee said. “Ultimately, this work should help provide insights into treatment strategies for AD treatment by regulating Aβ’s and tau’s effects.”
Lee added the NIH grant application will not be successful without the collaborative efforts from both his and Yao’s PhD students. Tao Teng helped to create the preliminary data with the blood-brain barrier model, and Taehoon Kim and David Le helped with the retinal OCT/OCTA imaging and the processing of Alzheimer’s disease mice.